-Sounds simple, right?
It is not as easy as you may think for a cloud droplet to grow larger.
-
First, lets
take a look at the size of a typical cloud droplet
in
comparison to a typical rain droplet:

Diagram made based on Ahrens 1994.
A typical rain drop is about
1 million times larger than a typical cloud droplet!!!
BUT WAIT! Even cloud
droplets need to start somewhere right?
It all begins with water
vapor. As you may already know, moisture is all around
us in the atmosphere in the form of water vapor.
You cannot see water vapor, it is the
gaseous form of water and is one of the most variable gases in the atmosphere.
In Tropical areas it can account for up to 4% of the atmosphere (by volume)
and in much drier areas, such as deserts, it can account for much less
than 1% of the atmosphere.
Water vapor requires special
conditions in order to form a cloud droplet of liquid water:
-
Supersaturation
is
one condition, but is not an easy condition to meet. Supersaturation
is defined in Meteorology as:
"The condition existing
in a given portion of the atmosphere (or other space) when the relative
humidity is greater than 100%, that is to say, when it contains more water
vapor than is needed to produce saturation with respect to a plane surface
of pure water or pure ice".
-
The predominant, and more effective
way to form a cloud droplet involves a tiny particle or aerosol,
known as a condensation nucleus.
Condensation nuclei can be made of natural materials such as dust or volcanic
ash, or can be made of man-made pollutants such as smoke particles.
-
There are as many as 1000 condensation
nuclei in one cubic centimeter of air!


Diagram made based on Lutgens 1992.
-
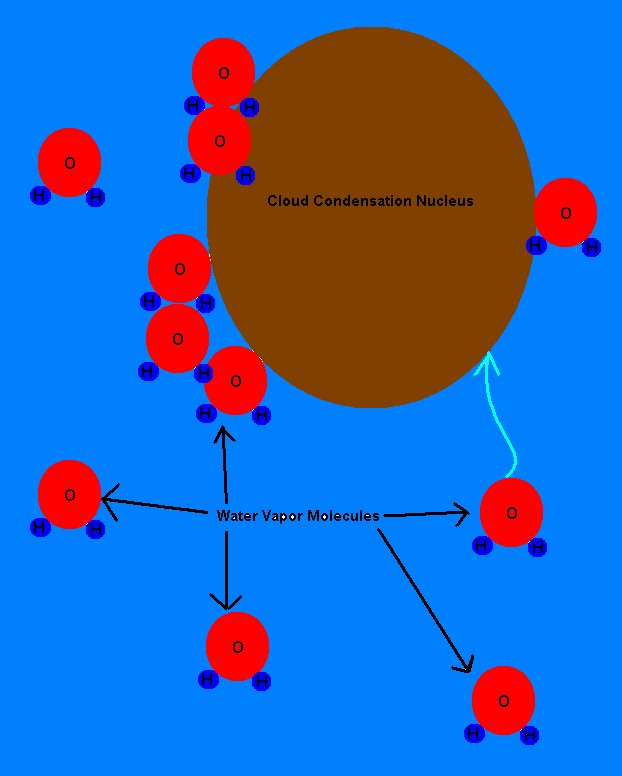
These cloud
condensation nuclei (CCN)
are very important and very effective, because they serve as surfaces on
which water vapor can condense.
As air from the surface
of the earth rises, it cools through a process called Adiabatic
expansion. Adiabatic expansion
occurs when a parcel or large volume of air is heated at the earth's surface
so that it becomes lighter and less dense than the surrounding air.
Since the air is now less dense than its surroundings, it is able to rise
up, as long as surrounding air is cooler and more dense, to heights of
several thousand to tens of thousands of feet above the earth's surface.
Air near the surface is more dense than air higher up because gravity holds
most of the molecules and particles of air in the atmosphere down near
the surface. [This also explains why the atmospheric pressure is
greater near the surface; because there is a more air above and yes, air
does have a mass and therefore weight to contribute to pressure!]
Now as this air rises up into the atmosphere where the pressure is less
and the air is less dense, there are much fewer particles of air moving
around and bouncing off from each other. [When particles of air move
around and bounce off each other, they are said to exchange kinetic
energy, or "energy of motion".
This exchange of kinetic energy is what releases heat and causes the sensible
temperature (that you can feel) of the air to be warmer. Since the
effect of gravity is less at higher altitudes, the air is able to expand
and move more freely where there are not as many particles to collide with.
The air then cools because there are not as many collisions and exchanges
of kinetic energy. If the air is not yet at 100% relative humidity,
it will cool at the dry adiabatic lapse rate
of 18 degrees F per 3280 feet. If the air is saturated (at 100% relative
humidity) it will cool at the moist adiabatic lapse
rate of around 9 degrees F per 3280 feet. When the air cools,
its ability to hold moisture decreases and the water vapor is condensed
out. That is to say warm air holds more water vapor than cold air.
Picture a hot "muggy"
summer day and a cold, snowy winter day: each one of these days
may have a relative humidity of close to 100% but because the summer day
is warmer, the air contains much more moisture and you can feel the wetness
in the air. A snowy winter day, on the other hand feels drier because
the cold air holds much less moisture. 100% Relative Humidity simply
means that the air is holding its maximum amount of moisture for a particular
temperature.
Therefore as the air
becomes colder, water vapor must condense into liquid water because the
air cannot hold as much water vapor as it could just moments before when
it was at a higher temperature. The water vapor molecules condense
on the surface of the CCN until the
air is in equilibrium and there are no more water vapor molecules available
for condensation without making the air surrounding the drop too dry.
Before you know it, a cloud droplet has formed. In fact, many billions
of cloud droplets can form in a matter of minutes causing a large cloud
to form.
* NOTE: Without cloud
condensation nuclei, supersaturation must reach relative humidities
of several hundred percent in order for water vapor molecules to condense
freely!!!
Click
here to continue!
to continue!
Click
here for the Main Precipitation
Page!



